Hi readers, have we got a treat for you this week.
It’s no secret that there is a substantial shift (finally) in the feline business space. We’ve heard that 2025 is the:
Year of the Cat
Decade of the Cat
Era of the Cat (though this last one sounds overly messianic).
Last week, we spoke to Alex Chieng, CEO of Pawsible Ventures, an early-stage pet-first fund and incubator. Here’s what he had to say:
“I definitely have [seen a shift in terms of investment opportunities towards cats]. And I know private equity investors are going down towards that trend. So I'm excited to get involved in the feline space … Out of these startups that I've met with so far, about a good 20-25% are in the feline space.”
Another sign of progress lies in therapeutics.
Today, we are really excited to bring you our exclusive interview with Gallant, a biotechnology company on the cusp of receiving FDA approval for the world’s first allogeneic stem cell therapy for cats.
We spoke with Dr. Rebecca Windsor, Gallant’s Director of Veterinary Affairs about what this means for the treatment of refractory chronic feline gingivostomatitis (FCGS), a devastating disease that potentially affects 1 in 4 domestic cats worldwide.
This immune-mediated disease causes extremely painful inflammation affecting the oral cavity, making it too painful to eat. Current treatment is typically full teeth extraction; cats who don’t respond to this often face euthanasia.
Lastly, we bring you a first-person account from feline-focused media personality KJ, whose beloved cat Grayson developed FCGS. Despite the odds, Grayson survived and is now living his best cheeky life. Read her heartfelt account below.
Question for you: Have you had to deal with FCGS? Would you consider stem cell therapy?
In the news
A turning point for feline medicine: Gallant’s bid to make stem cell therapy mainstream
Dr. Rebecca Windsor, Gallant’s Director of Veterinary Affairs, granted Feline Business Brief an exclusive interview
California-based Gallant is preparing for what could be the first-ever FDA approval of an allogeneic stem cell therapy for cats
Conditional FDA approval is anticipated for the first half of 2026

Dr. Rebecca Windsor
At Feline Business Brief, we strive to bring you closer to the breakthroughs shaping the cat industry. But few interviews feel quite as momentous as this one.
Gallant is on track to make history next year, with what could be the first FDA-approved allogeneic stem cell therapy for cats. The treatment targets refractory feline chronic gingivostomatitis (FCGS), a devastating disease that potentially affects 1 in 4 domestic cats worldwide.
If approved, it would mark a milestone not just for Gallant, but for veterinary medicine as a whole.
“This is a brand new category in veterinary medicine,” Windsor told Feline Business Brief. “Ready-to-use stem cells. It’s a shift from symptom management to targeting the disease at the source.”
The FDA’s conditional approval pathway is designed for conditions, like FCGS, with few or no effective treatment options. Conditional approval would bring the therapy to market while Gallant completes a larger follow-up study, paving the way for full approval.

Carolyn Wrightson, PhD, Chief Technical Officer at Gallant.
FCGS: A debilitating disease
FCGS is brutal. Cats develop an overactive immune response, leaving their mouths inflamed, with lesions and ulcers so painful they often stop eating. The cause remains uncertain, and 1 in 3 cats do not respond to traditional therapies.
The most common remedy is full mouth extraction (complete extraction of all the cat’s teeth). Even then, survival is far from guaranteed.
“For those cats that don’t respond to extractions, the options are limited, and unfortunately euthanasia is sometimes the only outcome,” Windsor said.
Prevalence of FCGS vary widely, from an estimated 0.7%-1% of cats to 26%.
”That’s hundreds of thousands of cats in the US alone,” Windsor said. “Because this is a chronic disease with no good alternatives, a ready-to-use stem cell therapy could be a true breakthrough for the cats suffering from it.”

Robin (left) enjoying life again.
Robin’s story
One of Gallant’s most powerful case studies is Robin, a cat who had reached the point of euthanasia. Nothing had worked for poor Robin, who was in intense pain and unable to eat.
After receiving stem cell treatment as part of Gallant’s FDA pivotal study, Robin was able to eat better. His pain subsided, and he was able to live a normal life. Not only did he improve, Robin is now in remission from refractory FCGS.
“We have so many stories of cats that were on the brink of euthanasia, that got cell therapy, and it completely changed their lives,” said Windsor.
“Cats that you saw have some of those lesions, huge ulcers and masses in their throats that are gone. They're normal cats now, after suffering for years.”
Why stem cells?
Stem cells work by calming an overactive immune system, reducing inflammation, and promoting healing. “Stem cells have high anti-inflammatory properties with tissues, they act directly as immunomodulators, they regulate a dysregulated immune system, and they promote tissue healing,” Windsor said.
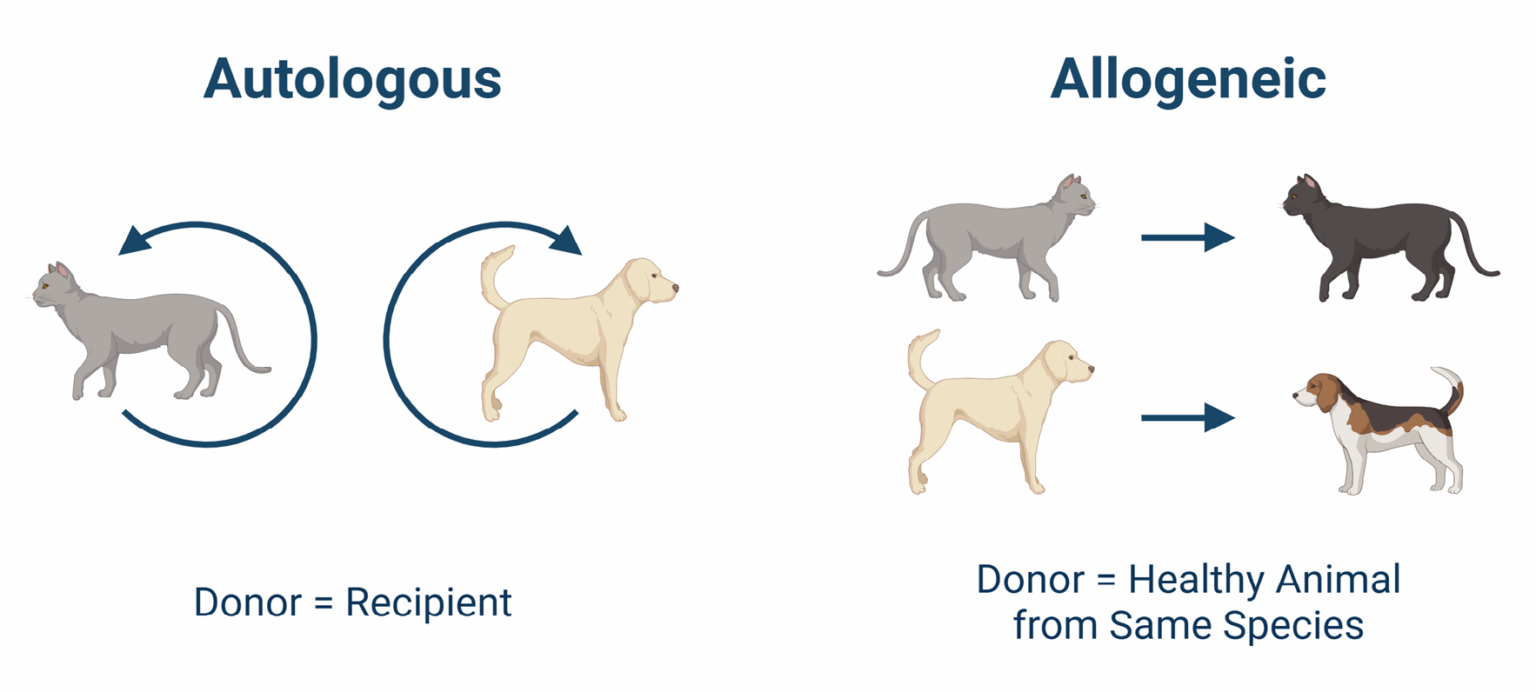
Notably, Gallant’s therapy uses allogeneic mesenchymal stem cells derived from the uterus of young, healthy donor animals.
“These are very high-quality sources of cells, and when you get them from young animals, they are more potent,” Windsor said. “Allogeneic means we can create an off-the-shelf solution, rather than having to collect and process cells from each individual patient. That makes treatment much more scalable.”
This is in contrast to autologous stem cells, where each patient must provide their own cells.

Chris Osborn, Biomanufacturing Associate at Gallant, handling cryogenic materials inside Gallant’s GMP-grade lab.
Normalising stem cell therapy
Stem cell therapy for cats might once have sounded like science fiction. But with Gallant’s FDA submission around the corner, off-the-shelf stem cell treatment may soon become reality.
Stem cells could also offer an alternative to long-term pain medication and immunosuppressive therapy, which can be costly over time.
“The nice thing with stem cell therapy is you’ve got two treatments, given two weeks apart, and that’s it. If you add up all the costs of daily, monthly, yearly medications and blood work, it probably adds up to more than two doses of stem cells.”
Closing the education gap
Windsor is quick to point out that regulation is only half the battle. Misconceptions and lack of education about stem cell therapies are common.
“Right now, even across veterinary conference circuits, there are no regenerative medicine tracks,” she said. “You might get a lecture here and there, but it’s not consistent. That’s why education is a big initiative for us.”
Gallant is preparing a free learning platform dedicated to stem cells, as well as a push to get more content onto conference agendas.
“Our hope is that, over the next couple of years, stem cell education will be mainstream,” she said.
“A lot of what hinders people from being able to make recommendations for their patients is that they don't feel comfortable talking about it. So if they can't field questions, if they don't feel like they understand what stem cells are or the basic science of how they work, then they don't feel comfortable recommending it to the pet parents. There's this big gap there.”
Education is also vital in helping vets and pet parents distinguish between high-quality, regulated therapies and products of questionable origin.
“Until we are in a situation where we have FDA-approved allogeneic products, it’s really on the veterinarian to be aware of what they are being sold,” Windsor said. “There’s a lot of stuff out there. Our responsibility as a profession is to really know what we’re doing, what we’re getting, and be able to tell the differences.”
Gallant’s stem cells go through extensive testing to verify their quality and potency.
Looking ahead: beyond FCGS
While Gallant’s FCGS candidate is nearest FDA approval, the company is also developing treatment candidates for other diseases. Gallant’s pipeline includes candidate treatments for feline and canine osteoarthritis, feline chronic kidney disease (CKD), and canine atopic dermatitis.
Osteoarthritis, Windsor said, is a particularly urgent area.
“Upwards of 90% of older cats have osteoarthritis. Because the way they manifest their symptoms is so different than dogs, I think oftentimes veterinarians don't even recognise it,” Windsor said. “Despite how common this disease is, cats are still underrepresented.”
“Our ultimate hope is to just change the entire mindset of the way that veterinarians think. To try to think, the first thing I should try to do is to see if I can heal this disease, rather than only managing symptoms.”
For Windsor, this work is personal as well as professional. She shares her life with Mango, her own cat. “I do feel like my bond with her has gotten closer because I’ve had to become so much more aware of feline health and understanding feline manifestations of pain. I can’t even imagine if she had FCGS.”
That bond, she said, reflects what’s at stake for millions of pet parents. “At the end of the day, these are patients and pets and people’s loved ones … That’s what drives us.”
Busting myths on stem cells
Gallant’s investigational treatment uses uterine-derived allogeneic mesenchymal stem cells (collected from carefully screened donor animals) to regulate the dysregulated immune system behind the disease.
Unlike autologous therapies, where each patient must provide their own cells, the allogeneic approach enables a ready-to-use solution at scale. Stem cells work also quickly. “They [stem cells] go straight into the body and start acting almost immediately.”
Created in BioRender
Myth 1: Autologous and allogeneic stem cells are the same
Reality: They’re fundamentally different. Autologous cells (taken from the patient) vary widely in quality, potency, and composition, often containing only a small percentage of actual stem cells. By contrast, allogeneic “ready-to-use” stem cells are derived from screened healthy donors, manufactured under strict GMP standards, and deliver consistent, predictable results.
Myth 2: Stem cell therapy has no oversight
Reality: Allogeneic stem cell products are regulated by the FDA’s Center for Veterinary Medicine and classified as drugs. For serious conditions like FCGS, conditional approval pathways already exist, allowing earlier patient access while ensuring safety and efficacy.
Myth 3: IV stem cells just get trapped in the lungs
Reality: While many MSCs do initially localise in the lungs, this isn’t a dead end. In the pulmonary environment, they activate anti-inflammatory pathways and then migrate to target organs (liver, spleen, heart, even brain) guided by inflammatory signals. They also secrete cytokines that influence immune responses systemically.
Myth 4: Allogeneic stem cells are rejected by the immune system
Reality: Immune reactions to MSCs are rare. These cells are “immune-evasive”, i.e. lacking MHC II and expressing minimal MHC I, meaning they avoid detection and remain effective without triggering rejection responses.
Myth 5: Stem cells can form tumours
Reality: The MSCs used in veterinary therapy are multipotent (not pluripotent or totipotent) and lack the ability to generate tumours. They support healing, not organ or tissue regeneration through uncontrolled cell growth.
Myth 6: Stem cells are only a last-resort treatment
Reality: Stem cells offer targeted, early disease intervention, not merely symptom relief. They modulate the immune response, reduce inflammation, and support tissue repair. This makes them a compelling option for conditions like osteoarthritis, CKD, and immune-mediated diseases, potentially more effective when used earlier.
Myth 7: Stem cells are harvested from embryos, causing ethical concerns
Reality: Veterinary stem cells are sourced from adult tissues from healthy donors. Gallant’s approach, for example, harvests cells from uterine tissue obtained non-invasively during routine procedures, offering ethical, consistent, and sustainable sourcing.
Myth 8: Stem cell therapy is prohibitively expensive
Reality: While commonly perceived as costly, single-treatment cost often compares favourably to long-term medication regimes. Most therapies involve just two injections, spaced two weeks apart - offering a potentially more economical and convenient alternative to ongoing therapy cycles.
Myth 9: Special equipment is needed to administer stem cells
Reality: Administering allogeneic stem cells is like a slow IV injection. Gallant’s therapy arrives frozen, is thawed in-clinic, diluted with saline, and administered via a standard catheter.
Source: Gallant
Grayson’s story

Grayson chilling at home. Photo courtesy of KJ.
Grayson came into our lives on International Batman Day. We named him after Robin’s alter ego, Dick Grayson - a sidekick, a survivor, a little superhero. He strutted across the room, sneezy and scrappy, like he’d already chosen us.
But behind that brave front was pain. A few months after adopting him, a dental specialist told us he had stomatitis. “We’ll have to pull his teeth,” the vet said. I asked, "Which teeth?" He replied, "All of them."
My husband and I were stunned.
After being reassured, cats live perfectly fine without their teeth, we clung to the hope he’d be one of the lucky ones who recover completely.
He wasn’t. Weeks later, he was still in agony. He stopped eating. He stopped playing. We had the appointment scheduled to let him go. My heart shattered.
And then we found Dr. Olden-Stahl - an integrative vet who looked at him and said, “I don’t let cats live in pain like this. Let’s make a new plan.” Steroids, laser therapy three times a week, acupuncture, herbs, supplements… little by little, Grayson fought his way back.
Now his tongue pokes out like his own superhero signature badge of courage. He not only survived, he went on to become a TV star, charming audiences with that silly tongue and resilient spirit. Grayson is my reminder that even in the darkest moment, sometimes the story isn’t over.
-KJ, Host of KJ TODAY and author of Raised by Cats: Behind the Mic and the Meows.
That’s it for now, thanks for reading! 🐾
P.S. We’re always interested in what you think! Is there anything in particular you’d like to share? Just reply to this email (or visit our Linkedin page), we’d love to hear from you.
